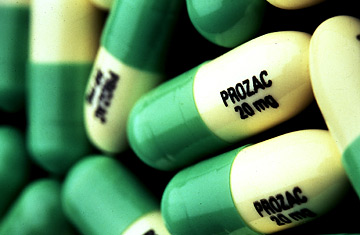
Did Prozac Cause Teenager to Kill? Psychiatrist Says Yes
“There is no reason other than a Prozac reaction,” said Dr. Peter Breggin, a New York state-based psychiatrist and author of the book, Talking Back to Prozac. “(The killing) is a mystery without that.”
Nine days after starting therapy with the drug, the teen attempted suicide via an overdose of his grandfather’s pills. His parents reported the incident to doctors, who increased the Prozac dosage for the teen.






SHARE YOUR STORY/COMMENT: