PR Newswire – December 21, 2011
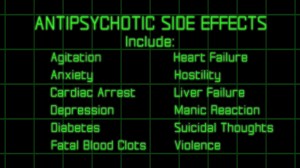
(Click image for international warnings on antipsychotic drugs) The "dear doctor" letter, sent to more than 7,000 doctors across South Carolina, and the package insert were found to be misleading about the safety and effectiveness of the antipsychotic drug Risperdal.
The jury verdict in the case of State of South Carolina versus Ortho-McNeil-Janssen Pharmaceuticals and Johnson & Johnson, Inc. has been upheld and requests for a new trial denied, affirming groundbreaking $327 million in civil penalties against the manufacturers of the drug Risperdal.
Circuit Court Judge Roger Couch announced the rulings on December 20 through two written orders. One order denies the defendant’s motion for judgment notwithstanding the verdict or, in the alternative, for a new trial; the second order denies the defendant’s motion to alter or amend the judgment and/or for a new trial. John B. White, Jr. and Donald C. Coggins, Jr. of Harrison, White, Smith & Coggins, P.C., a Spartanburg-based law firm, along with John Simmons of the Simmons Law Firm, a Columbia-based law firm, and Bailey Perrin Bailey, a Texas based law firm represented South Carolina in the case.
“We are obviously very pleased with Judge Couch’s decision and his careful consideration of this matter,” stated John B. White, Jr. one of the attorneys representing the state in the case. “The verdict handed down by the jury is just and speaks the truth. The damages awarded further substantiated the level of deception Janssen used in business practices in our state. Once again, we have sent a clear message to drug companies that deceptive business practices will not be tolerated in South Carolina.”
On March 22, 2011 a jury in the Spartanburg Court of Common Pleas found that New Jersey-based Janssen willfully violated the South Carolina Unfair Trade Practices Act by engaging in unfair or deceptive acts or practices in the conduct of any trade or commerce in the “dear doctor” letter of November 10, 2003 and the drug label (package insert). This decision represents the first jury verdict that finds the defendant violated unfair trade practices since the inception of its pharmaceutical product. The “dear doctor” letter, sent to more than 7,000 doctors across South Carolina, and the package insert were found to be misleading about the safety and effectiveness of the antipsychotic drug Risperdal. Risperdal was introduced by Janssen in 1994 and by 2005, generated annual revenues in excess of $3.5 billion.
On June 3, 2011 civil penalties amounting to $327,073,700 were ordered by Circuit Court Judge Roger Couch based upon violations found with the drug labels and “dear doctor” letters. Regarding the drug label violations, the judge ruled that 509,499 package inserts were distributed with sample boxes, and levied $300 per violation for a total drug label awarded damages of $152,849,700. Regarding the “dear doctor” letter violations, the judge ruled that 7,184 letters were mailed and 36,372 were provided during sales calls, and levied $4000 per violation for a total “dear doctor” letter awarded damages of $174,224,000.
The combination of the drug label and letter damages of $327,073,700 amounts to the highest verdict brought against Janssen for the drug Risperdal.
http://www.marketwatch.com/story/record-breaking-327-million-verdict-upheld-in-janssen-case-and-request-for-new-trial-denied-2011-12-21

